
120 million people—more than a third of Americans—will be diagnosed with cancer sometime in their lives, and more than 550,000 Americans will die of cancer this year. A&G’s theranostics platform addresses a large and underexplored opportunity: To identify cancers at a far earlier stage in the disease process— before tumors betray symptoms—and treat them with more targeted, personalized therapeutics that improve outcomes and save lives.
A&G Theranostic (Rx-Dx) Development:
Earlier Detection & More Effective Targeted Treatments
Overview
A&G’s approach is to develop linked diagnostic-therapeutic products based on unique, cancer-specific biological targets, or biomarkers, discovered using the company’s proprietary biomarker discovery platform. We expect our Rx-Dx tandems will enable more effective individualized treatment strategies and improve outcomes by empowering physicians to:
▲ TOP
A&G Companion Diagnostics:
Detect and monitor levels of cancer-specific biomarkers discovered by A&G
The chances of patient survival are significantly greater if cancer is diagnosed while still confined to the site of origin. Survival rates tend to decline precipitously as tumors enlarge and subsequently metastasize to other parts of the body. A&G is developing its proprietary cancer-specific protein biomarkers as tests that detect and monitor cancer from tissue and blood samples. The goal is to provide earlier, more reliable, clinically relevant information at substantially higher detection rates and specificity than traditional diagnostic tests. Our tests go beyond conventional diagnostic products that simply screen or confirm the presence of disease, with the potential to predict risk of disease, move initial diagnosis to an earlier stage of disease, stratify patients for clinical trials, and monitor therapeutic response. A&G’s molecular companion diagnostic tests are positioned to become critical components of cancer disease management and to improve therapeutic outcomes.
▲ TOP
A&G Targeted Therapeutics:
Engineered to Precisely Target the Unique Type of Tumor or Disease Cell
The most established types of targeted therapies are monoclonal antibodies and small-molecule inhibitors. While A&G pursues development of both types of medicines, our near- and medium-term efforts focus on monoclonal antibodies targeting cancer.
A&G Monoclonal Antibody-Based Therapeutics
We design our biomarker-derived monoclonal antibody (MAb) cancer therapeutics to block the proliferation of cancer cells by interfering with specific molecules that are required for tumor development and growth. By focusing on molecular and cellular mechanisms that are specific to the cancer, our novel approach produces medicines with unique therapeutic effect. Our products have the potential to be more effective and produce fewer side effects compared with conventional cancer treatments such as chemotherapy and radiation. Because these standard treatments are very non-specific and cannot distinguish between cancer cells and normal cells, they produce unwanted side effects and thus limit the amount of treatment that may be given at a given time.
▲ TOP
Advancing a New Treatment Paradigm
A&G’s lead theranostics program illustrates how co-development of a targeted therapeutic with companion diagnostic tests advances a powerful new treatment approach positioned to move diagnosis to a far earlier stage and dramatically improve outcomes in cancer treatment.
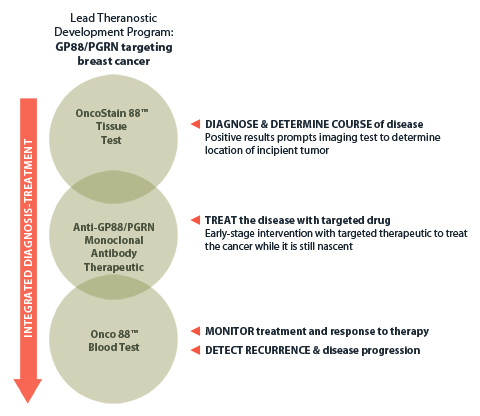 A&G Integrated Dx/Rx Product Integration
A&G Integrated Dx/Rx Product Integration
▲ TOP
Earlier Detection & More Effective Targeted Treatments
Page Contents
Overview
A&G Companion Diagnostics
A&G Targeted Therapeutics
Advancing a New Treatment Paradigm
Overview
A&G’s approach is to develop linked diagnostic-therapeutic products based on unique, cancer-specific biological targets, or biomarkers, discovered using the company’s proprietary biomarker discovery platform. We expect our Rx-Dx tandems will enable more effective individualized treatment strategies and improve outcomes by empowering physicians to:
- Detect disease at an earlier stage
- Identify patients who have a high risk of recurrence and mortality
- Select optimum treatment strategy on an individual patient basis
- Treat the biological mechanisms fueling disease progression
- Monitor patient response to the targeted therapy
- Identify when disease has become resistant to a certain treatment
- Monitor disease recurrence
▲ TOP
A&G Companion Diagnostics:
Detect and monitor levels of cancer-specific biomarkers discovered by A&G
The chances of patient survival are significantly greater if cancer is diagnosed while still confined to the site of origin. Survival rates tend to decline precipitously as tumors enlarge and subsequently metastasize to other parts of the body. A&G is developing its proprietary cancer-specific protein biomarkers as tests that detect and monitor cancer from tissue and blood samples. The goal is to provide earlier, more reliable, clinically relevant information at substantially higher detection rates and specificity than traditional diagnostic tests. Our tests go beyond conventional diagnostic products that simply screen or confirm the presence of disease, with the potential to predict risk of disease, move initial diagnosis to an earlier stage of disease, stratify patients for clinical trials, and monitor therapeutic response. A&G’s molecular companion diagnostic tests are positioned to become critical components of cancer disease management and to improve therapeutic outcomes.
▲ TOP
A&G Targeted Therapeutics:
Engineered to Precisely Target the Unique Type of Tumor or Disease Cell
The most established types of targeted therapies are monoclonal antibodies and small-molecule inhibitors. While A&G pursues development of both types of medicines, our near- and medium-term efforts focus on monoclonal antibodies targeting cancer.
A&G Monoclonal Antibody-Based Therapeutics
We design our biomarker-derived monoclonal antibody (MAb) cancer therapeutics to block the proliferation of cancer cells by interfering with specific molecules that are required for tumor development and growth. By focusing on molecular and cellular mechanisms that are specific to the cancer, our novel approach produces medicines with unique therapeutic effect. Our products have the potential to be more effective and produce fewer side effects compared with conventional cancer treatments such as chemotherapy and radiation. Because these standard treatments are very non-specific and cannot distinguish between cancer cells and normal cells, they produce unwanted side effects and thus limit the amount of treatment that may be given at a given time.
▲ TOP
Advancing a New Treatment Paradigm
A&G’s lead theranostics program illustrates how co-development of a targeted therapeutic with companion diagnostic tests advances a powerful new treatment approach positioned to move diagnosis to a far earlier stage and dramatically improve outcomes in cancer treatment.

▲ TOP



© 2009 A&G Pharmaceutical, Inc. All Rights Reserved. | Terms of Use | Privacy
